All Posts in "Health"
Access Holdings Steps Up Sickle Cell Awareness
Mohammed Shosanya
Employees of Access Holdings Plc,have donated 159 pints of blood as part of an initiative to commemorate Sickle Cell Awareness Month.
The donation drive,which saw over 200 employees participate, was executed in partnership with the Lagos State Blood Transfusion Service and the Boat Foundation/Life Bank.
This contribution will directly support sickle cell champions, who often require urgent blood transfusions due to life-threatening complications from the disease,a statement said.
Amaechi Okobi, Chief Communications Officer at Access Holdings PLC, spoke on the significance of the initiative, stating, “Every pint of blood donated represents hope and survival for someone living with sickle cells. We are incredibly proud of our staff who volunteered and our partners who made this possible. Access Holdings is committed to championing causes that change lives and create lasting impact.”
Besides,Access Bank, the flagship subsidiary of Access Holdings, hosted a webinar, featuring a distinguished panel of speakers, including Prof. Ebele Uche, Professor of Haematology at the Lagos State University College of Medicine; Dr. Bodunrin Osikomaiya, Executive Secretary of the Lagos State Blood Transfusion Service; Dr. Sola Adebekun, Executive Secretary of the Temitayo Awosika Help Foundation, and Ms. Judith Ojonugwa Mathew, a sickle cell champion.
The webinar focused on raising awareness about the challenges faced by individuals living with sickle cell and emphasised the importance of regular blood donations in managing the disorder.
The panellists shared insights on healthcare gaps, the need for increased blood supply, and the impact of public-private partnerships in supporting sickle cell patients.
The event also included personal stories from sickle cell champions, highlighting their daily struggles and the importance of timely interventions like blood transfusions.
Access Holdings’ involvement in this campaign underscores its dedication to advancing healthcare initiatives that benefit vulnerable communities.
Cholera:Oyo Confirms 27 Cases,Records One Death
Malaria Treatment:FG Bans Monotherapy Drugs
Mohammed Shosanya
The use of mono therapy for malaria treatment remains prohibited in the country,the National Malaria Elimination Program ( NMEP) of the federal ministry of health has said.
Program officer of NMEP, Wudi Natasha Tanko,who stated this during a media chart organized by the organization in Abuja,said monotherapy refers to the use of only one antimalarial medicine to treat malaria such as artesunate and artemeter injection, chloroquine and sulfadoxine/pyrimethamine (SP).
She said, ” monotherapies are not recommended for treatment of uncomplicated malaria and cannot cure. Chloroquine and SP are failed drugs with 39% and 56% efficacy respectively. Artesunate and artemeter injection have very short half live. They attack malaria parasite fast but do not sustain in the body to mop up residual parasites.
“Use of mono therapies for uncomplicated malaria is abuse.”
According to her,dangers of the use of mono therapies is incomplete cure, increased disease severity and development of drug resistance.
She stated that Artemisinin-based combination therapies ( ACTs) are the recommended treatments for uncomplicated malaria. She added that ACTs are medicines consisting of an artemisinin derivative and another schizonticidal antimalarial medicine.
She also said malaria cannot be diagnosed based on clinical (signs and symptoms) assessment alone , adding that diagnosis requires testing confirmation of a clinical suspicion.
She said: ”Case management intervention through prompt diagnosis and treatment with recommended antimalarial is pivotal for reduction of malaria burden.”
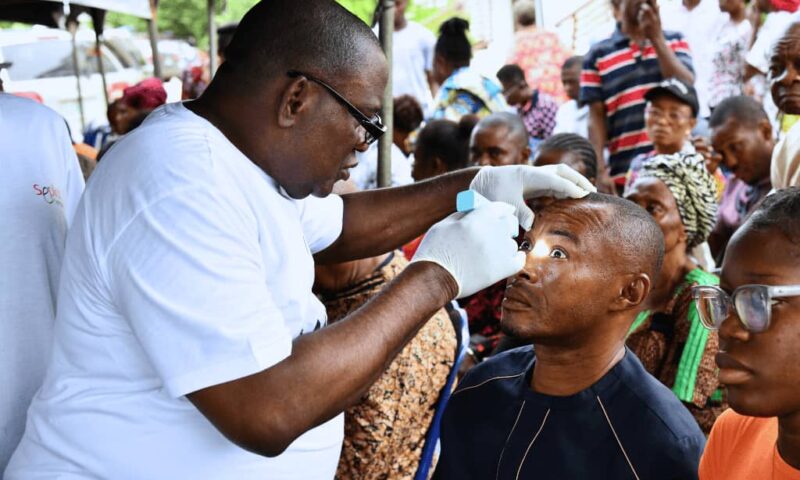
NNPC/Seplat Medical Outreach Programme Restores Vision In Imo
Mohammed Shosanya
The NNPC Ltd/ Seplat Energy Joint Venture (JV) partnership has conducted a medical outreach, providing free eye health services to individuals with visual impairments in Ohaji/Egbema community of Imo State.
Through its “Eye Can See” programme, a Corporate Social Responsibility (CSR) initiative, the JV dispensed more than 10,000 reading glasses and successfully performed 639 eye surgeries, including cataract removals, for host community members who otherwise had limited access to such vital medical services, a statement signed by Olufemi Soneye,Chief Corporate Communications Officer,said.
The “Eye Can See” programme, which commenced in 2017, has been a beacon of hope in the eastern asset of the NNPC upstream investments, positively impacting over 20,000 people to date.
Speaking during the event,Chief Upstream Investment Officer of NNPC’s Upstream Investment Management Services (NUIMS), Bala Wunti, represented by Dr. Obinna Otuu, Manager, JV Asset B emphasized the significance of the initiative to NNPC Ltd’s corporate mission of enriching the lives of Nigerians.
Wunti stated that the NNPC Ltd takes pride in being more than just an energy provider.
“We are a partner in progress, dedicated to making sustainable contributions to the communities that support us,” he added.
According to him, the “Eye Can See” initiative reflects “our belief that corporate structures can and should play a vital role in societal development.”
He noted that the programme goes beyond immediate medical care by educating individuals on lifestyle choices to prevent conditions like hypertension and diabetes, which can lead to permanent vision loss.
Expressing his appreciation for the support of the local government, beneficiaries, and NNPC Ltd’s partners, Wunti observed that together with Seplat, the National Oil Company is paving the way for a brighter future where access to essential health services is possible for all.
“This project is not just about restoring vision; it is about giving people hope and the opportunity to lead fulfilling lives. This year’s outreach in Ohaji/Egbema is a testament to the ongoing commitment of NNPC and Seplat to improve the quality of life in their host communities,” he affirmed.
The “Eye Can See” initiative has had a profound impact on the communities it serves. By providing free eye screenings, surgeries, reading glasses, and health education, the programme has transformed lives and restored hope to many who had been suffering from visual impairments.
6575 People Benefit From SNEPCo Vision First Plus Programme In Bariga LCDA
Mohammed Shosanya
No fewer than 6575 people benefitted from an eyecare outreach held in Bariga Local Council Development Area (LCDA) of Lagos State, August 26—30, as part of the Vision First Plus programme of the Nigerian National Petroleum Company Limited (NNPC) and Shell Nigeria Exploration and Production Company (SNEPCo).
Beneficiaries from within and outside the LCDA received a wide-range of medical services including eye screening, surgeries, medication, and eyeglasses on the latest campaign to bring healthcare to the doorsteps of communities across Nigeria, a statement said.
Speaking at the opening ceremony, SNEPCo Managing Director, Elohor Aiboni, said the outreach was a key investment in maintaining healthy vision which is an important but often neglected aspect of life.
Represented by Managing Counsel Upstream Nigeria, SNEPCo, Lara Taiwo-Ogunbodede, Aiboni commended the collaboration which resulted in the outreach, the third in Lagos since the Vision First Plus initiative was launched in the state two years ago.
She said:“Our sight, a crucial connection to our surroundings, safeguards us from danger, sharpens our minds, and unlocks the doors to learning, economic opportunities, and independence, hence we consider this intervention as crucial.”
In a speech read by Bunmi Lawson, the Chief Upstream Investment Officer of NNPC Upstream Investment Management Services (NUIMS), Bala Wunti, said: “From the smiles of individuals who can see clearly for the first time in years, to the stories of renewed hope and confidence, Vision First has truly become a beacon of light for many. This year, as we continue our journey, our mission remains unchanged: to ensure that no one is left in the dark due to preventable or correctable vision issues.”
The Executive Director and Chairman, Kolmarg Eyesight Foundation, Professor Olukorede Adenuga stressed the need for “regular eye examination as one gets older in order to prevent avoidable loss of vision.”
The Chairman of Bariga LCDA, Alabi Kolade, described the vision outreach as a timely intervention at a time that vision impairment is found to affect the quality of life of adult populations, lower people’s the rates of employment and increase their rates of depression and anxiety. “Sight is life, and it’s for this reason that we cannot thank NNPC-SNEPCo and the partners enough for this opportunity, indeed on our doorstep.”
Giving a breakdown of the programme performance, Aiboni said that out of 1787 people screened for various eye conditions, 1,696 received prescription glasses and medication while 233 had successful cataract and pterygium procedures.
Another 1212 people were treated for ailments.
Permanent secretary, Lagos State Ministry of Health, Olusegun Ogboye, who was represented by Adeniran Ifeyemi, A deputy director in the Ministry of Health, commended NNPC, SNEPCo, and their co-venture partners for the initiative.
The focus on eyecare comes against the background of a report in the National Eye Health Policy 2019 to the effect that blindness in three out of four people in Nigeria is preventable. The Vision First Plus programme aims to combat avoidable visual impairment by reaching patients early for diagnosis and treatment.
Over the years, the social investment programmes of NNPC, SNEPCo and co-venture partners have improved lives in internally displaced camps in Northeast Nigeria and rebuilt infrastructure in hospitals and educational institutions. In addition, the initiative has led to award of scholarships and donation of cancer treatment
Family Planning: UNFPA Seeks Accelerated Domestic Funding
Mohammed Shosanya
The United Nations Fund for Population (UNFPA), Nigeria Country Office has advocated the need for accelerated domestic funding in order to meet the Global Family Planning Commitments
It described family planning commodity as a key deliverable to achieving Nigeria ‘s family planning target of 27% MCPR by 2030,adding that the target might not be realized unless Nigeria meets up with her commitment by end of August 2024.
The UNFPA Programme Associate, Adegbotolu Oladipo,stated this while giving an overview of the National Basket Fund and the State based financing Tracker at a 3-day technical workshop on the development of Bauchi State based financing Tracker for family planning services organized by the Bauchi State ministry of health, State Primary Health Care Development Board with support from UNFPA held in Jo’s, Plateau State.
He said that for four years,Nigeria has not disbursed funds for the procurement of FP commodities in the National Basketball Fund, despite making a commitment of $4 million yearly contribution.
“In 2022-2023 Nigeria did not benefit from UNFPA supplies partnership matching fund of approximately 1.5million US Dollars yearly and is in the verge of missing 2024 match fund of 2million dollars”.
“Also for 2024, Nigeria did not receive from the second tranche of commodities worth 6 million dollars from UNFPA partnership of 347.709 million dollars.”
” There is potential of losing the third tranche approval if the commitment is not full fill by end of August 2024,” he said
He maintained that in 2024, the total commitment to FP is put at 40.755.218.40 million dollars out of which UNFPA made a commitment of 8.7 million dollars and has so far allotted 10.1 million .
He noted that the funding gap for 2024 stands at 24 million dollars and this accounts for the stock out of family planning commodities
Impact of the funding gap,he said, is estimated to lead to over 800.000 unintended pregnancy, 10.280 maternal deaths and some 340 unsafe abortions.
He urged Bauchi State government to make provisions for funds for the procurement of FP commodities.
He said,states like Lagos, Ogun, Delta,and of recent Adamawa and Rivers have made contributions for the procurement of commodities for their states.
He stated that an average cost of FP commodity per woman stands at 3.2 dollars, noting that the return on each dollar is is 69.3 dollars.
Bauchi State Commissioner of Health, Dr Adamu Sambo represented by Director Medical Services, Dr Suleiman Abubakar said that the financing tracker will help government monitor FP funds.
Executive Chairman, State Primary Healthcare Development Board, Dr Rilwanu Mohammed pointed out the need for health related MDAs to unbundle their sources of funding.
Nigeria Must Strengthen Healthcare Regulatory Institutions-NESG
Nigeria Must Strengthen Healthcare Regulatory Institutions-NESG
Mohammed Shosanya
The Nigerian Economic Summit Group (NESG) has advocated the need to strengthen healthcare regulatory bodies in Nigeria.
The group gave the advice at a pre-summit webinar in preparation for the 30th Nigerian Economic Summit (#NES30),with the theme, “Strengthening Healthcare Regulatory Bodies in Nigeria”.
At the summit,gathered experts and stakeholders discussed the challenges within Nigeria’s healthcare regulatory framework as well as develop actionable recommendations for enhancing regulatory capacity to ensure effective healthcare regulation across the country.
Speaking,Dr. Oyebanji Filani, Ekiti State Commissioner for Health and Human Services, and Steering Committee member of NESG’s Health Policy Commission (HPC), emphasised the crucial role of a strong healthcare system in national development.
He spoke on the significant impact that well-resourced regulatory bodies can have on public safety, investor confidence, and overall healthcare quality in Nigeria.
According to him,addressing current challenges urgently is essential to fostering an environment conducive to healthcare development, which in turn supports socio-economic advancement.
In his keynote address,Dr. Abdu Mukhtar, National Coordinator of the Unlocking Healthcare Value-Chain Initiative, noted that healthcare currently contributes 3-4% of Nigeria’s GDP, a stark contrast to 17% in the United States and 15-20% in Europe.
He emphasized the necessity of a robust healthcare system for national stability and competitiveness.
He also outlined the government’s ongoing healthcare reform plan, anchored on four pillars such as effective governance, improved population health outcomes, unlocking the healthcare value chain, and enhancing health security, particularly in pandemic preparedness.
He said the plan,which covers 2022-2026, includes cross-cutting themes such as regulation, financing, and digitalisation.
He further discussed the Presidential Initiative to unlock the healthcare value chain, launched in October 2023.
He explained that,the initiative involves 20 key regulatory agencies and five ministries and aims to increase local pharmaceutical production, reduce medical tourism,and attract investment.
Significant achievements, according to him, include a presidential executive order removing duties on machinery and materials for medical product manufacturing and the signing of several Memoranda of Understanding (MoUs) to support research and development platforms for vaccine production and to strengthen regulatory bodies.
The stakeholders at the event highlighted the challenges facing Nigeria’s healthcare regulatory bodies, such as limited budgets, insufficient staffing, and outdated laws.
These issues,they said,hinder thorough inspections, compliance enforcement, and the overall effectiveness of regulatory oversight, posing risks to patient safety.
The National Health Policy (2016) also pointed out the absence of an institutional framework to regulate quality and standards, and the lack of robust enforcement mechanisms that allows unqualified individuals to practice medicine illegally.
At the panel discussions,
Dr. Victor Gbenro, Deputy Registrar, Medical and Dental Council of Nigeria (MDCN), underscored the role of MDCN in regulating medical education and upholding standards in medical training and practice across Nigeria.
She also addressed resource gaps that hinder the council’s ability to double the number of medical graduates.
Professor Mojisola Adeyeye, Director General of the National Agency for Food and Drug Administration and Control (NAFDAC),emphasised the importance of local pharmaceutical manufacturing.
She stated that manufacturing 70% of pharmaceuticals locally and importing 30% would allow for better monitoring and adherence to good manufacturing practices.
She assured that NAFDAC would continue to conduct routine and targeted inspections to ensure these standards are met.
Ms. Modupeola Ogundimu, Director, Lagos Zone, National Health Insurance Authority (NHIA), pointed out the challenges facing health insurance in Nigeria and opportunities for improving the regulatory environment to promote Universal Health Coverage (UHC).
She also discussed the impact of recent economic hardships on health insurance and the steps needed to strengthen the NHIA’s regulatory mandate.
Dr. Ajibike Oyewumi,Healthcare Quality Specialist, IFC, emphasised the importance of enhancing regulatory capacity through training, technology adoption, and establishing strong public-private partnerships.
She also noted the critical role of national health insurance in providing funding to ensure access to quality healthcare.
Dr. Abiola Idowu, Executive Secretary, Health Facilities Monitoring and Accreditation Agency (HEFAMAA): highlighted HEFAMAA’s mission to enforce healthcare facility standards and accreditation in Nigeria.
She emphasized the need for increased investment in technology, training, and collaboration with stakeholders to improve transparency and public engagement in healthcare regulation.
Dr. Ameer El Telwany, CEO, Egypt Healthcare Authority (represented by Ms. Nermeen Ashour), shared insights on the reforms and system changes implemented by the Egypt healthcare system.
The webinar event underscored the importance of strengthening healthcare regulatory bodies to ensure a transparent and well-regulated healthcare sector in Nigeria.
The NESG affirmed that improved regulatory frameworks are essential for attracting domestic and foreign investment, enhancing the global competitiveness of African healthcare industries, and fostering regional integration and trade in healthcare products and services.
Oyo Govt Urges Healthcare Centres To Expedite Action On HIV/AIDS Eradication
Mohammed Shosanya
The Oyo State Government, through the Oyo State Agency for the Control of AIDS (OYSACA) has urged Healthcare Centres providing Comprehensive HIV treatment, care and support to People Living with HIV (PLHIV) across the State to enhance their performance.
The agency’s Chairman, Dr. Gbola Adetunji,gave the charge recently during a working visit to some treatment sites in the state.
He stated that the agency is conducting the visits to familiarize herself with the centres, identify challenges, and determine areas where OYSACA can provide assistance.
He admonished the centres to maintain a friendly demeanor towards PLHIV and frequently educate them that being HIV positive does not mean the end of life and emphasized that PLHIV can live healthy long lives if they adhere to treatment and follow prescriptions.
He advised individuals to regularly check their HIV status using free and voluntary testing facilities available at Primary Health Care Centres (PHCs), State Healthcare Centres, and select Private Healthcare Centres cut across the State. The Chairman stressed further that HIV testing is the only means to confirm their status.
In their separate responses, the ART Coordinators at the visited centres appreciated the Agency’s timely actions and steps, appealing to the Chairman to address the issue of inadequate test kits facing some centres, facilitate and resuscitate of support groups of PLHIV accessing treatment and care in their facilities.
They thanked Governor Seyi Makinde for appointing Dr. Gbola Adetunji as OYSACA Board Chairman, believing his wealth of experience would enhance the agency’s activities and visibility.
The visited sites included Comfort Medical Centre, Total Garden, Idi-Ogungun Primary Health Care Centre, Agodi Gate, Adeoyo Maternity Teaching Hospital (AMTH), Yemetu all located in Ibadan.
WHO Gives NDDC Plaudit For Tackling Cholera Outbreak
Mohammed Shosanya
The World Health Organisation, WHO, has commended the Niger Delta Development Commission, NDDC for taking proactive measures to address the challenge of a looming cholera outbreak in the Niger Delta region.
Its Country Representative to Nigeria, Dr Walter Kazadi Mulombo, gave commendation while handing over a partnership framework to the Managing Director of the NDDC, Dr Samuel Ogbuku, that will drive the development of the region during a courtesy visit at the NDDC headquarters in Port Harcourt.
According to a statement signed by Seledi Thompson-Wakama, Director, Corporate Affairs, Mulombo also commissioned 13 ambulances acquired by the NDDC for distribution to hospitals in its nine mandate states.
He noted that most countries in the world were lagging behind in meeting the United Nations Sustainable Development Goals, SDGs, especially in the area of healthcare.
He said:“There is an urgent need to tackle the global climate change challenge because it brings about various health issues, one of which is cholera outbreaks.
“I congratulate the NDDC for organising free healthcare missions in the Niger Delta region and I look forward to optimising the collaboration we have established so far. We are also preparing to organise a health summit in the region.”
Responding, the NDDC Managing Director, Dr Ogbuku, said that the first phase of the Commission’s free healthcare mission, concluded recently, was very successful as many people benefitted from the programme.
He added: “The free medical outreach has been one of the flagship programmes of the Commission, with documented evidence and abounding testimonies of its beneficial impact in enhancing the quality of life of the rural poor in the region.”
“This programme, which provides healthcare services to medically underserved rural communities in the region, is one of several health programmes of the Commission, targeted at changing the health situation and narrative of our people in the region. It is in line with the United Nation’s Sustainable Development Goals, SDGs, No. 3 which aspires to achieve universal health coverage and ensure health and well-being for all.
“We have successfully treated many medical conditions, including eye surgeries and the distribution of corrective eyeglasses to aid patients in reading small prints and improving their vision.
“Patients have also received various medications for conditions such as malaria, hypertension, diabetes, sexually transmitted diseases (STDs), gastrointestinal disorders, dental care, surgeries for conditions like appendicitis, as well as diagnostic services including, random blood sugar tests.”
Ogbuku said that because of the high demand for the health services, the NDDC decided to carry out the health mission twice a year, rather than once.
The NDDC boss said that the WHO was discussing with the Commission to be part of the free health programme, as well as other health programmes that will benefit the people of the Niger Delta region.
“The participation of WHO will add professionalism and credibility to what we are doing in our medical outreach programme. It will also ensure that those vaccines that we don’t have access to, are procured through them for the benefit of our people.
“We are not only looking at what they will bring to us in terms of funding, we are looking at their contacts, reach and expertise in the medical field.”
Speaking during the commissioning of the 13 new ambulances, the Chairman of the NDDC Governing Board, Mr. Chiedu Ebie, appealed to the benefiting hospitals to put the ambulances to good use in the service of Niger Deltans.
He commended the WHO for partnering with the NDDC in developing the health sector, noting that the NDDC could not do it alone.